
Introduction
Despite best efforts with physical therapy, medicines, pain interventions, and surgeries, some patients will have persistent neuropathic leg or arm pain or back pain. In some situations, spinal cord stimulation may decrease pain in these areas and improve quality of life.
What is spinal cord stimulation?
Spinal cord stimulation has been used for decades for the treatment of chronic neuropathic lower extremity pain in patients whose pain has not improved with spine surgery. Over the last several years, improvements in technology have led to new and better stimulation patterns that have improved and expanded the use of this technology while making it more convenient for patients.
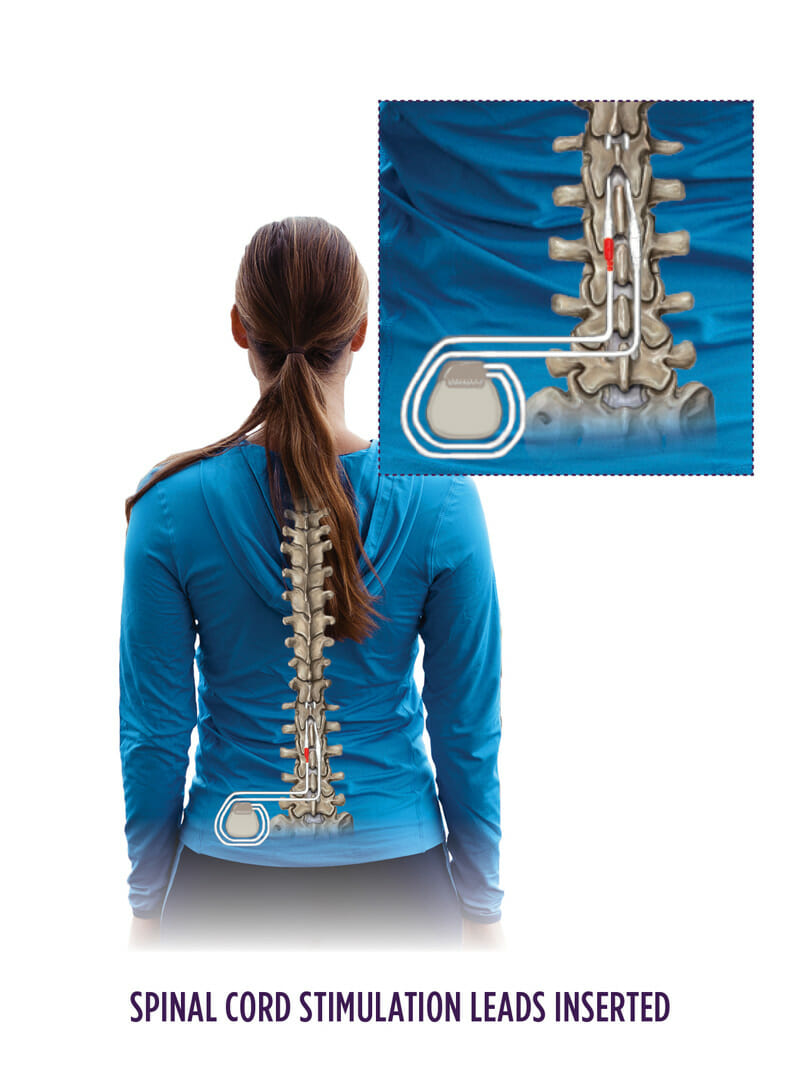
Stimulator leads are placed in the epidural space near the mid to lower spinal cord in the middle back (thoracic spine). There, stimulation is applied to the spinal cord. Depending on the device used, you may feel a paresthesia (a buzzing or massaging sensation) overlapping the area of pain you have, or in some devices, no sensation at all. The goal of therapy is to decrease pain and improve function.
A significant advantage of spinal cord stimulation is that there is a trial period before the device is permanently implanted. This way the patient can try the technology and experience its effect. The patient then has a reasonable degree of certainty as to what kind of pain relief he or she would obtain with a permanent implant.
Who is a candidate for spinal cord stimulation?
If you are interested in spinal cord stimulation or believe you could benefit from the procedure, please speak with your Summit specialist. It is generally recommended for:
- Patients with chronic, neuropathic extremity pain (burning or electric sensations)
- Some patients with chronic low back pain
Overview of the spinal cord stimulator trial procedure
Here’s what to expect during the spinal cord stimulator trial procedure:
- You will lie face down on a special X-ray table.
- The skin over the injection site is cleaned, prepared, and numbed.
- You will be given medicine to make you feel relaxed and comfortable.
- Using fluoroscopic (X-ray) guidance, your physician will guide a specialized needle into the epidural space.
- Your doctor will insert a spinal cord stimulator lead through this needle and guide it to the appropriate position.
- Your doctor tests the leads in the procedure room.
- The leads are secured with a specialized dressing and covered with medical tape.
The trial period lasts about one week.
- During this time, you should try to keep the area dry and clean. Sponge baths are permitted, but you can’t shower or submerge the dressing.
- A device representative will work closely with you during the week in order to optimize pain control.
- You will then be seen about one week later in the office, where the specialist will remove the trial leads, help determine if the trial was successful, and provide further guidance.
How should I prepare for the spinal cord stimulator trial procedure?
- Follow the specific instructions given to you by the nurses at the procedure center.
- While the procedure usually takes around 30 to 45 minutes to perform, you should allow for at least one to two hours at the procedure center.
- If you are taking prescription blood thinners such as Coumadin (warfarin), Ticlid (ticlopidine), or Plavix (clopidogrel bisulfate), please inform your patient care coordinator. These medicines will need to be stopped before the procedure, but only after you receive permission from the doctor who is prescribing these medicines.
- If you are on high doses of aspirin (more than two per day), tell your doctor’s patient care coordinator.
- Tell your doctor’s patient care coordinator if you have a pacemaker.
- If you develop a fever, night sweats, or an active infection before your procedure, your procedure will need to be rescheduled. Please contact our office at (651) 968–5201 immediately to let us know about the symptoms you’re experiencing.
Potential risks of spinal cord stimulator trials
Spinal cord stimulator trials are relatively safe, minimally invasive procedures. The risks associated with the procedure include:
- Bleeding
- Infection
- Pain at the injection site
- Trauma to nearby structures from needle or lead placement
These complications are all very rare.
After the procedure
- Follow the specific instructions given to you by the nurses at the procedure center.
- Plan to rest for a few hours. You may resume light activity that is comfortable for you, but do not overexert yourself the first day.
- For discomfort, apply ice packs to the area for 15 minutes several times a day.
- Do not take a tub bath or shower. You may take a sponge bath while the trial lead is in place.
- Report any signs of infection or other unusual symptoms to our office, including:
- Redness and warmth at the injection site
- Increasing pain
- Swelling or drainage at the injection site
- Chills, night sweats, or fever that reaches above 100° F
- Keep a record of your pain and symptoms during the week of your spinal cord stimulator trial. The device representative will be in close contact with you throughout the trial period. You will then follow up with your specialist in clinic in approximately one week to have the leads removed and discuss your response to the trial period.
If your procedure includes sedation
- You should have no solid foods for six hours before your procedure.
- You may have clear liquids up to two hours before your procedure. Examples include:
-
- Water
- Broth
- Clear fruit juices such as apple, cranberry, and grape juice (no pulp)
- Tea, black coffee with no cream
- Carbonated beverages
- Nothing by mouth, including throat lozenges, mints, and all hard candy, for two hours before your procedure.
- No gum for two hours before your procedure.
- You must have a responsible adult arrive with you to our facility and drive you home. If you use a taxi or volunteer ride service, you still must have a responsible adult with you in order to help take care of you after your procedure.
- Please take your regular medications the day of your procedure, especially any heart, diabetes, or blood pressure medications.
Summit Orthopedics offers comprehensive spine expertise
Summit’s spine care team is recognized by the National Committee for Quality Assurance for the comprehensive expertise of our patient-centered care. Our back specialists diagnose spine problems and design custom treatment plans built on a conservative, nonsurgical approach. Most patients find relief through treatments including guided injections, specialized physical therapy, biofeedback, exercise, activity modification, and medication. When conservative care does not relieve symptoms, our highly skilled surgeons offer proven, evidence-based surgical options. Together with you, we will determine the right course of action.
Start your journey to a healthy spine. Find your spine expert, request an appointment online, or call us at (651) 968–5201 to schedule a spine consultation.
Summit has convenient locations across the Minneapolis-St. Paul metro area, serving Minnesota and western Wisconsin. We have state-of-the-art centers for comprehensive orthopedic care in Eagan, MN, Vadnais Heights, MN, and Woodbury, MN, as well as additional community clinics throughout the metro and southern Minnesota.
More resources for you
- Visit our Spine Exercise Library for options to help ease neck and back pain
- See Summit’s treatment options for neck, back, and spine care
- Check out additional information on Summit’s approach to spine care
